
Written by

*UPDATE* The total number of regulatory approvals for AI solutions for medical imaging has increased since we wrote this insight. To receive the latest copy of our Database detailing regulatory-approved AI solutions for medical imaging, please click here.
11th July 2019 – Cranfield, UK – Written by Dr. Sanjay M. Parekh – Receiving regulatory approval is one of the final hurdles medical technology companies must overcome before commercializing their machine learning solutions. Following this, companies are then able to license the software solution for clinical use.
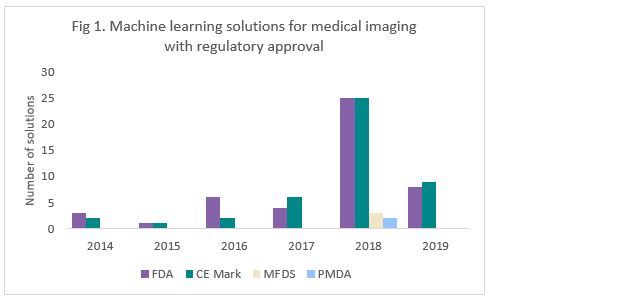
We have explored the companies which have received regulatory approval for their machine learning solutions since 2014, across four main markets: USA (FDA); Europe (CE mark); South Korea (MFDS); and Japan (PMDA).
A total of 45 machine learning solutions have received CE marking compared to 47 solutions that received FDA regulatory approval. However, for the Asian markets, there were only a total of 5 products that received either MFDS or PMDA approval, and none have received China FDA clearance. Figure 1 shows an exponential rise in the number of approvals granted in 2018, which coincidentally were the same for Europe and the USA (25 solutions each).
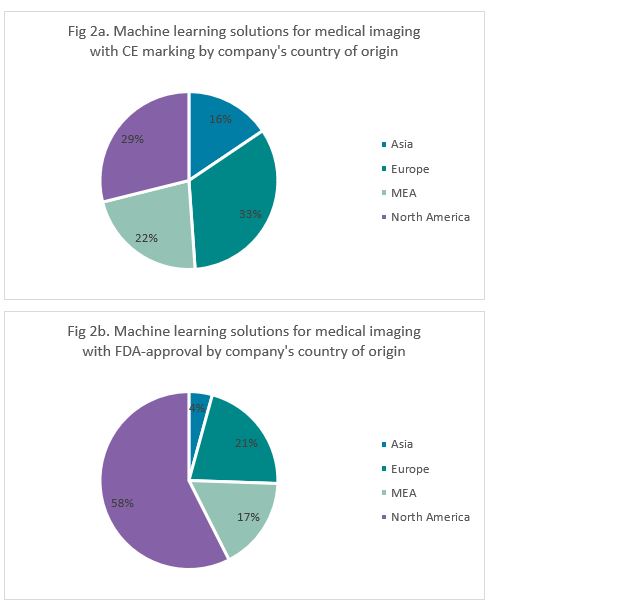
When exploring the countries where these companies were founded, we note that most (33%) European approvals (CE marking) were granted to companies founded in Europe (Figure 2a). The same was true for USA approvals (FDA), but the majority was far greater (58%) (Figure 2b). Regulatory-approved solutions from companies originating in Asia lagged compared to their Western counterparts for both CE marking (16%) and FDA-approval (4%). Companies from the USA and Europe have undergone robust clinical validation trials and tend to favour regulatory approvals from their home market in the first instance.
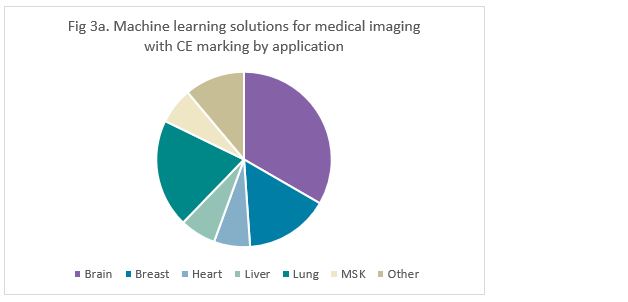
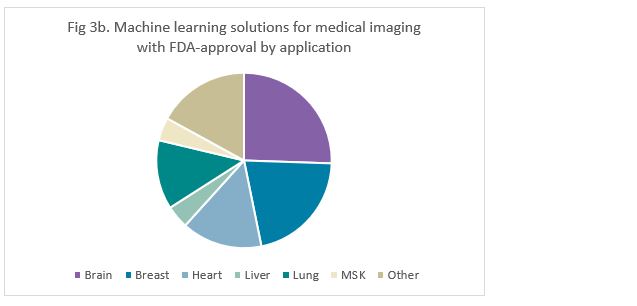
Most machine learning solutions with CE marking were targeted to the brain (33%), followed by the lung (20%) (Figure 3a). Similarly, solutions with FDA-approval were mostly targeted towards the brain (26%), but this was closely followed by breast solutions (21%) (Figure 3b).
The US FDA and PMDA (Japan) have recently signaled that the regulatory process for machine learning solutions for medical imaging will become more streamlined. Considering this, we anticipate that the number of regulatory approvals for machine-learning solutions targeted at medical imaging will continue to increase over the coming twelve months.

