Written by

Cranfield, UK, 29th March 2023 – End-tidal (Et) control has been utilised in the global premium anaesthesia market for almost a decade, however not all countries have managed to reap its benefits. The US, a pioneer and leader in healthcare provision, has only recently been exposed to the benefits this technology can offer with the launch of GE HealthCare’s Aisys CS with End-tidal Control in 2022. This insight discusses the use of end-tidal control and identifies the advantages it will offer the US market.
End-tidal Control Reduces Costs and the Environmental Impact of Anaesthetic Gases
As the need for clinical decision support grows and focus is placed on improving cost efficiency, awareness of the advantages of automated anaesthesia control is growing. In Europe, the use of end-tidal control has been utilised to improve patient care, while prioritising environmental impact. End-tidal control allows anaesthesiologists to set gas targets specific to the patient, promoting personalised and individualised care. Using ‚Äòbreath by breath’ data, and through interoperability with anaesthesia machines, specified gas targets are maintained despite any potential changes in a patient’s physiological status (see Figure 1). End-tidal control software can relieve anaesthesia staff of time-consuming tasks, such as the necessary but continuous adjustment of anaesthesia machines required to sustain optimum end-tidal control concentrations. Reduced touchpoints and automated care enable healthcare professionals to focus their attentions on quality patient care and observation, reducing clinical workload and risk of burnout.
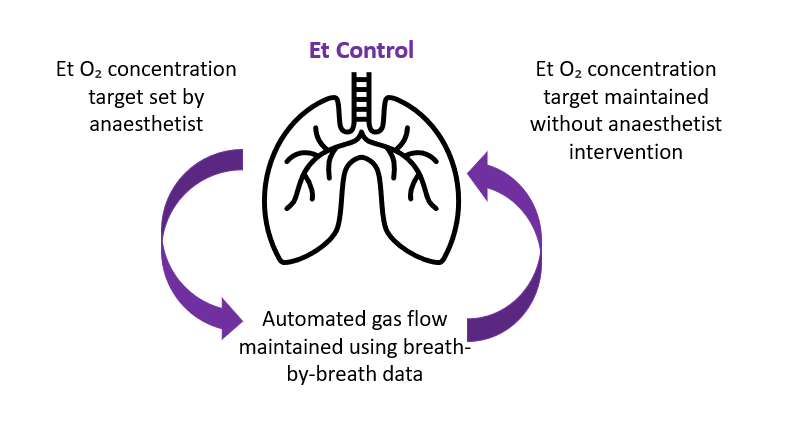
Figure 1
End-tidal control has set the precedent for anaesthesia gas control in many countries, providing valuable clinical decision support, improving clinical workflow, and reducing the emissions of anaesthetic gases. Evidence suggests the automated control of patient end-tidal control software can result in a reduction of greenhouse gas emissions of up to 44%. Improved efficiency and maintenance of anaesthetic gas release is largely responsible for these reductions, and with this improved efficacy, comes reduced costs. A reduction in almost one third in operating costs can be attributed to decreases in the amount of anaesthetic gas required per patient. With the escalating costs of anaesthesia gas delivery, the interest to reduce costs wherever possible is understandably of growing desire amidst healthcare providers.
Long-awaited US approval and release of GE HealthCare’s Aisys CS Software is Met with Further Roadblocks
The US launch of GE HealthCare’s Aisys CS² technology in 2022 has sparked the arrival of end-tidal control software into the market. FDA Pre-Market approval (PMA) of this software has been long awaited and is expected to be met with a multitude of economic, environmental, and patient-centred benefits, as seen in countries where end-tidal control has been utilised for some time.
Despite the array of benefits and functionality the GE HealthCare software offers, the technology has been met with many obstacles, many of which stem from a lack of awareness of the importance of end-tidal control and its environmental benefits in the US. To date, attitudes towards environmental sustainability have not been universal amongst regions. Western European countries have shown an eagerness to reduce CO² emissions and the release of harmful gases and waste products in healthcare. Unfortunately, the drive towards eco-consciousness has been slower in the US. Despite US hospitals pledging to reach net zero by 2050 as part of the UN’s Net Zero Coalition, action taken to reach this goal seems to be lacking.
The presence of GE HealthCare’s end-tidal control technology in the US is expected to improve sustainability awareness and promote further innovation in anaesthesia automated support. GE HealthCare is currently the sole vendor legally able to market end-tidal control software in the US. Other vendors in the global anaesthesia market are unlikely to rush to invest in this lengthy and costly regulatory procedure, given the significant training, education, and awareness required for the US market to see the benefits of their technologies. However, they are likely to observe and take note of the uptake from the side-lines.
Other limitations preventing vendors expanding their end-tidal market to the US include the extensive clinical trials necessary to gain PMA and FDA approval. The large installed base and clinical presence required to achieve this will undoubtedly prove difficult and require significant investment.
Uptake of this new technology by anaesthesiologists and hospitals in the US is likely to be slowed down by the labelling under the PMA that indicates the specific training or experience practitioners require to use the device. This training requirement subsequently appears to be a roadblock in the use of automated anaesthesia in the US, further echoing the significant investments in awareness and marketing required to create space in the US market for this software and an appreciation of the benefits it offers.
Future of Automated Anaesthesia Gas Control in the US
Despite the barriers mentioned, there is progress and improvement towards to the use of end-tidal control in the US, with growing interest in mitigating the environmental impact of anaesthesia delivery in addition to improving the overall healthcare experience.
However, it is likely that market growth and expansion in the US will be limited to GE HealthCare’s solution for the coming years. The educational and financial investments required to prepare the US for this technology will most probably reduce the likelihood of market penetration for many vendors. Despite this, the presence of end-tidal control across Europe, Canada, Latin America, and Asia continues to be highly promising and indicative of a global and sustained attitude towards environmental consciousness, economic awareness, and improved patient care.
About the Report
Signify Research’s upcoming report “Anaesthesia Devices Report – 2023” will build on their 2021-edition and will provide a data-centric and global outlook of the market. The report will blend primary data collected from in-depth interviews with healthcare professionals and technology vendors, to provide a balanced and objective view of the market.
About Signify Research
Signify Research provides healthtech market intelligence powered by data that you can trust. We blend insights collected from in-depth interviews with technology vendors and healthcare professionals with sales data reported to us by leading vendors to provide a complete and balanced view of the market trends. Our coverage areas are Medical Imaging, Clinical Care, Digital Health, Diagnostic and Lifesciences and Healthcare IT.
Clients worldwide rely on direct access to our expert Analysts for their opinions on the latest market trends and developments. Our market analysis reports and subscriptions provide data-driven insights which business leaders use to guide strategic decisions. We also offer custom research services for clients who need information that can’t be obtained from our off-the-shelf research products or who require market intelligence tailored to their specific needs.
More Information
To find out more:
E: enquiries@signifyresearch.net
T: +44 (0) 1234 986111
www.signifyresearch.net