Written by

Cranfield, UK, 25th March 2024 – Fetal monitors play a pivotal role in modern obstetrics, providing crucial information on fetal heart rate (FHR) and maternal uterine activity (UA). These electronic devices, which can be attached internally or externally, aid healthcare professionals in assessing fetal well-being during pregnancy and childbirth. The demand for ambulation during childbirth in developed countries has driven the need for wireless solutions, emphasising flexibility and personalised birthing experiences. However, challenges such as a lack of skilled workforce and varying levels of awareness and education have to date impacted the wider adoption of fetal monitoring, particularly in emerging regions.
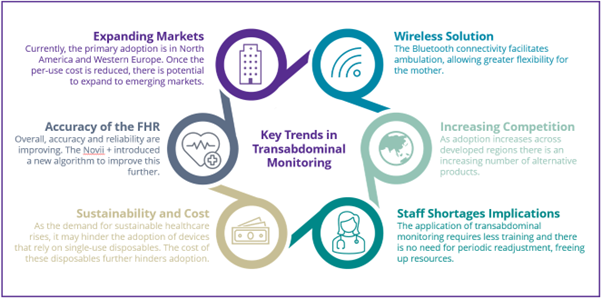
As a result of these conditions, the developed market has seen an increased uptake of transabdominal fetal monitors. These monitors employ electrocardiography for intrapartum monitoring, offering a one-time easy application. Despite their benefits, challenges such as high costs associated with disposable patches, sustainability concerns, and limitations in water use due to Bluetooth technology, hinder widespread adoption. Considering these challenges, what is the likelihood of sustained growth in adoption?
Tackling the underlying problems of standard fetal monitoring
Effective traditional fetal monitoring demands the expertise of healthcare professionals proficient in applying transducers correctly and interpreting results efficiently. The prevailing global shortage of healthcare professionals has intensified the challenges associated with monitoring capacity. In emerging regions, insufficient training further compounds the issue, hindering optimal utilisation of monitoring technologies. In transabdominal monitoring, an adhesive patch is affixed to the mother’s stomach, offering two primary advantages. Firstly, applying the patch doesn’t necessitate the presence of a healthcare professional, reducing the resource need. Secondly, unlike standard transducers, there is no requirement for periodic readjustment, streamlining the monitoring process and ensuring continuous, hassle-free usage.
In most recent studies, transabdominal monitors have proven more accurate than standard CTG monitoring for mothers with higher body mass indexes (BMIs). Companies like GE HealthCare and Philips offer these monitors, with uptake to date mainly concentrated across North America and Western Europe, where there is a more developed healthcare infrastructure. The potential for broader implementation exists, and overcoming barriers like limited compatibility with different brands could pave the way for advancements. Nevertheless, there remains apprehension regarding the dependability and precision, even though advancements are being made.
The potential solution, but at what cost?
Upon recognising the advantages, it becomes imperative to consider the challenges and limitations still facing transabdominal monitors and hindering its adoption.
The use of disposable patches in transabdominal fetal monitoring presents a double-edged sword in the pursuit of effective and convenient intrapartum monitoring. On the one hand, these patches offer a one-time, easy application, aligning with the demand for cableless solutions and enhancing user experience. However, the high per-use cost, approximately $80, poses a significant barrier to widespread adoption. Sustainability concerns also loom large, as the environmental impact of disposal solutions raises questions about the long-term viability of such an approach. Hospitals and clinics, particularly in resource-limited settings, or those serving economically disadvantaged populations, may find investing in a technology that relies on expensive disposable components financially burdensome. The cumulative expenses associated with frequent patch usage can also place further strain on healthcare budgets, potentially limiting the widespread implementation of transabdominal monitoring solutions.
As the technology advances, there is optimism that prices will decrease, hopefully making transabdominal monitors more cost-effective. Companies like GE HealthCare, Philips, Nemo Healthcare, Mind Child and others are actively contributing to overcoming these challenges. The balance between cost-effectiveness, sustainability, and ease of use will be pivotal in determining the future trajectory of disposable patches in transabdominal fetal monitoring, influencing their adoption and impact on maternal healthcare outcomes.
Furthermore, the cost considerations may extend to individual users, as patients may bear a portion of these expenses, affecting their willingness to opt for transabdominal monitoring over more traditional or affordable alternatives. To enhance the adoption of this technology, manufacturers and healthcare stakeholders must address the cost barriers associated with disposable patches, exploring avenues for cost reduction or alternative, more sustainable solutions.
The growth of ambulation during birth is a result of offering more flexibility and choice to pregnant mothers. Water births are becoming increasingly common; this method is praised for its potential to ease labour, reduce stress, and provide a gentle transition for the baby. Whilst a transabdominal monitor generally improves ambulation, its flexibility is limited as the Bluetooth connectivity of the pod which connects to the monitoring system is restricted within water.
The accuracy of transabdominal fetal monitors, while promising, encounters limitations influenced by external factors such as the use of oils on pregnant women’s stomachs.
Oils and creams, commonly applied for skincare, create a barrier that may interfere with the effectiveness of the monitor’s sensors. This interference can lead to inaccuracies in the data captured, posing challenges in providing reliable information on fetal well-being. As the demand for wireless, transabdominal solutions rises, addressing these practical challenges becomes imperative for broader adoption. Innovations aimed at mitigating the impact of external substances and ensuring consistent accuracy will be pivotal in enhancing the reliability of transabdominal fetal monitors and bolstering their utility in maternal healthcare.
Overcoming the challenge, somewhat:
Recent strides in transabdominal monitoring are exemplified by the announcement of GE HealthCare’s Novii+. The recent FDA-approval for the monitor with an expanded indication marks a positive development, showing some promise to overcome the initial barriers to adoption. The Novii+ features an enhanced algorithm, significantly improving reliability and accuracy. Moreover, the extended applicable timeframe for monitoring preterm patients from 34 weeks onward expands its utility. The progress made by GE HealthCare underscores the potential for innovation and growth in the field, showcasing how technological advancements can address limitations and enhance the effectiveness of transabdominal fetal monitors.
The road ahead:
With GE HealthCare’s new product development coming in 2024, as well as other vendors producing and developing new solutions in this space, there will no doubt be keen eyes on the potential success. Product innovations will continue to influence the market; however, some factors will impact the level of innovation going forward.
Stringent regulations, while crucial for patient safety, may hinder the development and adoption of transabdominal monitors. The need for compliance adds complexity to product development, requiring manufacturers to invest in research, testing, and adherence to standards. Striking a balance between regulatory requirements and fostering innovation is crucial for continued progress.
Disparities in technology adoption and healthcare infrastructure contribute to discrepancies between regions. While developed countries may lead in embracing wireless technology and advanced fetal monitors, emerging regions may still rely on more affordable alternatives like fetal dopplers. Bridging this gap requires addressing barriers such as price sensitivity, lack of training, and awareness of the benefits of continuous fetal monitoring. Despite this, as the upward trajectory of adoption in more developed markets continues, accessibility to other regions will likely increase overtime.
In conclusion, the product development of transabdominal fetal monitors, reflects a dynamic landscape with challenges and promising advancements. The progress made by companies like GE HealthCare indicates a positive trajectory, but overcoming barriers related to cost, compatibility, and regional discrepancies is essential for realising the full potential of fetal monitoring technology. As obstetrics continues to evolve, innovative solutions that prioritise both patient outcomes and accessibility will play a crucial role in shaping the future of fetal monitoring.
Related Research
Signify Research has recently published its “Fetal Monitor – World – 2024” report. This brand-new report provides a deep-dive analysis of the fetal monitor market. It covers the global trends impacting the market and coverage of the competitive landscape. The analysis is broken down to a product and regional level, including fetal monitor insight at a country level.
About The Author
Sam joined Signify Research in 2023 as a Market Analyst within the Clinical Care team. He brings experience working as an Economist for the Intellectual Property Office, creating strategy reviews and impact evaluations, used to aid government trade negotiations. Sam holds a first-class bachelor’s degree in economics from Loughborough University, where modules included topical issues such as healthcare. Away from the office Sam enjoys staying active with dog walks, football and cricket, and his interest in volunteering took him on a charity visit to Ethiopia.
About the Clinical Care Team
The clinical care team provides market intelligence and detailed insights on the clinical care equipment and IT markets. Our areas of coverage include patient monitoring, diagnostic cardiology, infusion pumps, ventilators, anaesthesia devices, and high-acuity IT. Our reports provide a data-centric and global outlook of each market with granular country-level insights. Our research process blends primary data collected from in-depth interviews with healthcare professionals and technology vendors, to provide a balanced and objective view of the market.
About Signify Research
Signify Research provides healthtech market intelligence powered by data that you can trust. We blend insights collected from in-depth interviews with technology vendors and healthcare professionals with sales data reported to us by leading vendors to provide a complete and balanced view of the market trends. Our coverage areas are Medical Imaging, Clinical Care, Digital Health, Diagnostic and Lifesciences and Healthcare IT.
Clients worldwide rely on direct access to our expert Analysts for their opinions on the latest market trends and developments. Our market analysis reports and subscriptions provide data-driven insights which business leaders use to guide strategic decisions. We also offer custom research services for clients who need information that can’t be obtained from our off-the-shelf research products or who require market intelligence tailored to their specific needs.
More Information
To find out more:
E: enquiries@signifyresearch.net
T: +44 (0) 1234 986111
www.signifyresearch.net